Aurobindo Implements TRI2 Application Object Migrator (AOM) which is a part of Triniti’s Application Lifecycle Management for Oracle E-Business Suite (EBS) and other GMP and non-GMP Applications as Part of GxP


Client Background
From a single unit manufacturing Semi-Synthetic Penicillin (SSP), Aurobindo Pharma is now the market leader in Semi-Synthetic Penicillins, with presence in key therapeutic segments such as neurosciences, cardiovascular, anti-retrovirals, anti-diabetics, gastroenterology and anti-biotics, among others.
"A big thank you for all the efforts put in by Triniti in deploying the AOM tool successfully at Aurobindo. Some of the key benefits that we are able to realize out of AOM are:
-
Paperless and error-free change control process. This replaced the tedious manual change control process
-
Ease of tracking and monthly review of closed change control records
-
Ageing report is helpful for all the functional leads to closely monitor change control records and for taking appropriate action
-
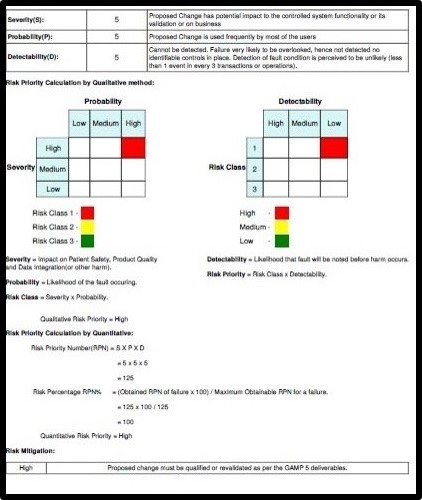
Auto Risk Assessment calculation provided is highly useful for the CSV/QA team
-
Auto Migration of objects to non-production and production environments
- Venkat M, GM, Information Systems
Outcomes
In addition to the benefits highlighted by Mr. Venkat from Aurobindo, these are the other outcomes:
- Reduced cycle time from change initiation to closure
- Enhanced productivity - improved visibility and centralized change administration
- Auto-generated Risk Assessment report needed for compliance as per GxP
- SOX-compliant change management process enforced by the application
- Governance made easy
- Single tool to track changes for GMP and Non-GMP applications
Opportunities/ Challenges
Aurobindo Pharma has varied enterprise applications to manage its organization-wide processes across different streams. These applications change in response to evolving business requirements. GxP related regulatory controls require stringent compliance in the Pharma industry. Hence, all application changes must be logged, approved, and validated. Manual change migration meant the following challenges:
- All application changes were logged, approved, and validated manually
- Multiple groups are involved in a series of activities for migrating the changes to various environments
- While complying with the defined process, teams focused on their part of the activity rather than having end-to-end visibility of the change. Stakeholders relied on emails/explicit communications to learn the progress and status of the change
- Long turnaround times for change deployment owing to the preparation of multiple documents manually
- Lack of a system to track or audit collaboration across business, IT, System Administration, and QA teams, resulting in longer turnarounds, low visibility
Solution Highlights
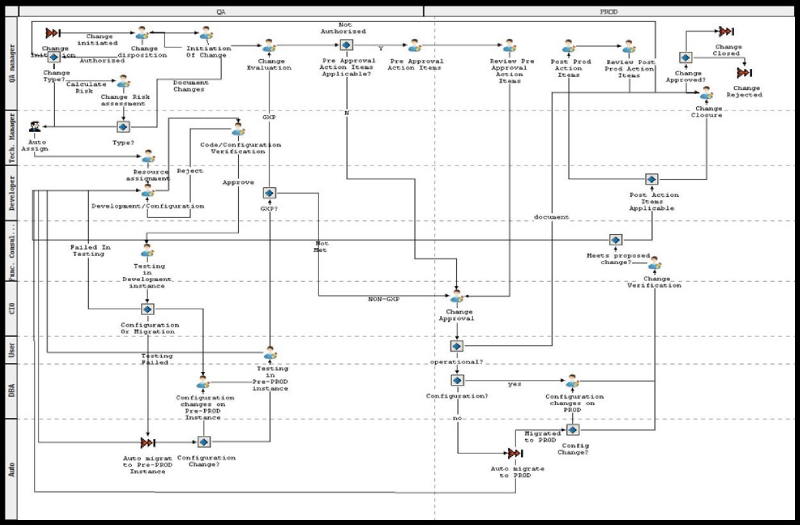
Triniti’s change management tool provides one interface to capture all activities, events and associate the resultant artifacts with the application change lifecycle. Finally, it helps transition the changes across the controlled development, QA and production environments. Some of the highlights of the solution implemented at Aurobindo are:
- Defined change management workflows for seven GXP applications and five non-GXP applications
- Made changes to the application to capture electronic signatures at approval events under CFR Part 11 requirements on Electronic Records and Electronic Signatures (ERES)
- In line with rigorous application validation as part of CFR Part 11 Compliance, Triniti facilitated Aurobindo teams to validate the AOM application for compliance with Installation Qualification, Operational Qualification, and Performance Qualification requirements
- Systematic enforcement of a strict password policy mandated by the FDA
- Established process for repositing change objects/code into the Version control system and integrated the same with AOM
- Routed approval notifications automatically based on application and change type, drawing stakeholders’ attention and capturing inputs
- Automated generating Risk Assessment Sheet and Change Control Record for each ticket based on information by the QA team
- AOM created migration documents saving time for developers and system administrators
- Controlled data access using role-based security
Change Management Workflow for Oracle EBS